Laboratory
When the collection has ended, take the semen to the lab for testing and preparation of the semen doses. The semen should be in the lab within 20 minutes of collection.
 |
 |
To prevent contamination, the pig pens and the laboratory need to be clearly separated. Make sure that the lab itself is a clean zone, where boots and clothes worn in the pens are not allowed. Wash and disinfect your hands and if necessary change into a clean set of clothes.
|
|
|
Analysis and registration
All semen collections must be analysed in the lab. This involves several steps to ensure the optimal quality and productivity of each collection.
Step 1 – Analysis of the semen
1. Visible analysis of the semen:
Look at the semen carefully to make sure it has a milky white colour.
The normal range for the colour of semen is from milky white to pale yellow (skimmed milk – heavy cream). If the colour is outside the normal scale, dispose of the sample because it could be contaminated with blood or urine.
|
|
|
2. Odour analysis:
Smell the semen. The smell should be neutral. If you detect the smell of urine components or any other unexpected odour dispose of the semen.

3. Microscopic analysis:
- Prepare a semen sample on a slide.
|
|
|
- Carefully lower a cover slip onto the slide and place the sample under the microscope on a heated surface.
 |
 |
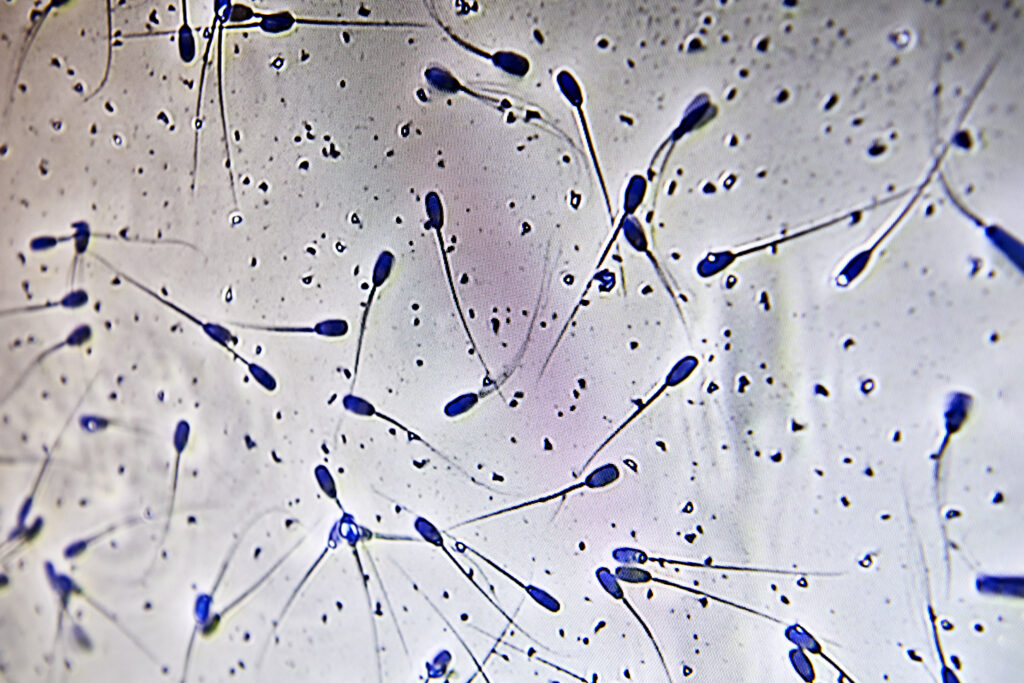
- Check motility of the sperm cells.
Look at the semen under the microscope and check for:
- Morphology (appearance) – normal sperm cells do not have deformities such as a bent tail, double tail, coiled tail, droplets (proximal/distal) or acrosomes.

- Motility (forward movement) – there are two ways to evaluate this.
- Visual evaluation: find a corner on the specimen, count 10 sperm cells and if 7 out of 10 are normal, the motility is 70% which is the minimum acceptable level.
 |
 |
-
- Calculation using CASA systems, which is connected to a PC and uses a digital camera to extract data from the semen.
 |
 |
4. Sperm count
Measure optical density with a colorimeter or photometer to determine the concentration of the semen doses.
- How concentrated is the semen?
- How many sperm cells per batch?
 |
 |
This method helps optimise the number of boars on the farm and leads to more homogenous and uniform doses.
Step 2 – Batch registration of the semen:
When the semen has been collected, all data associated with the produced batch is recorded under a unique batch number. Data includes the DanBred ID no., name of the boar, date and time of collection, volume and analytical profile.
 |
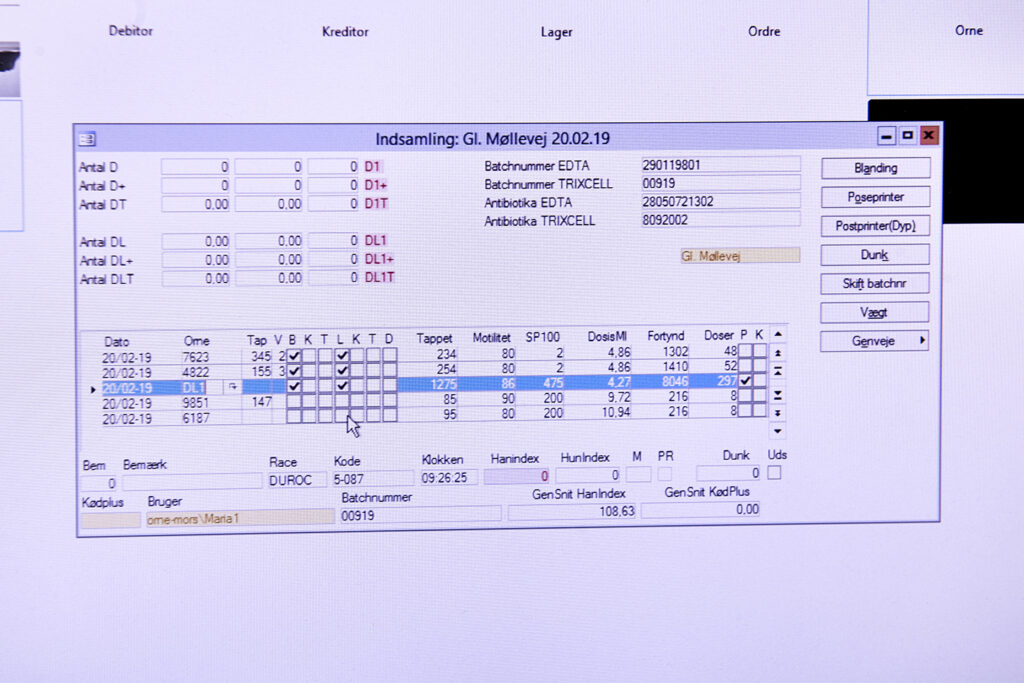 |
Semen for purebred inseminations must never be pooled, but should only contain semen from one boar. The boar ID no. or name, along with boar race, should be registered on the dose.
|
|
|
Production semen or semen from terminal boars can be pooled/mixed in a batch. Pooling semen from more boars can increase fertility rates. Use a minimum of 3 different boars when pooling semen to ensure the proper fertility effect. Note that pooled batches should be registered in the same way as purebred semen.
|
|
|
Dilution of the semen concentrate for AI application
When semen has been cleared for production, make a dose calculation based on the sperm count in the collected semen dose. Aim for a sperm count of at least 2.0 billion (109) cells per dose of terminal production semen and 2.1 billion (109) cells per dose for purebred semen.
The following steps should be completed before you start the dilution process:
1. Demineralized water is mixed with the semen extender according to the prior calculations.
 |
 |
2. Heat the solution of the demineralised water and extender to 30-35°C in a water bath. Add the solution to a plastic bag or can, and place it in the water bath for a minimum of one hour or until the extender is fully dissolved.
|
|
|
| Facts |
|---|
| The choice of extender will determine the shelf life of the semen. For example, LT-Extender and TRIXcell+ will give a shelf life of 7 days for pooled terminal semen but only 3 days for purebred semen. Shelf life is calculated from the day of collection and mixing. |
 |
 |
3. Remove the plastic bag containing the semen from the plastic container. Use a pair of disinfected scissors to open the corner of the plastic bag and pour it into the 35°C mixture of demineralized water and dilution.
 |
 |
4. Take a sample of the diluted semen, and perform quality control of the finished semen solution to ensure that sperm is still viable.
 |
 |
5. Fill bottles/bags with the semen solution to be used for the insemination. This can be done manually or by machine.
 |
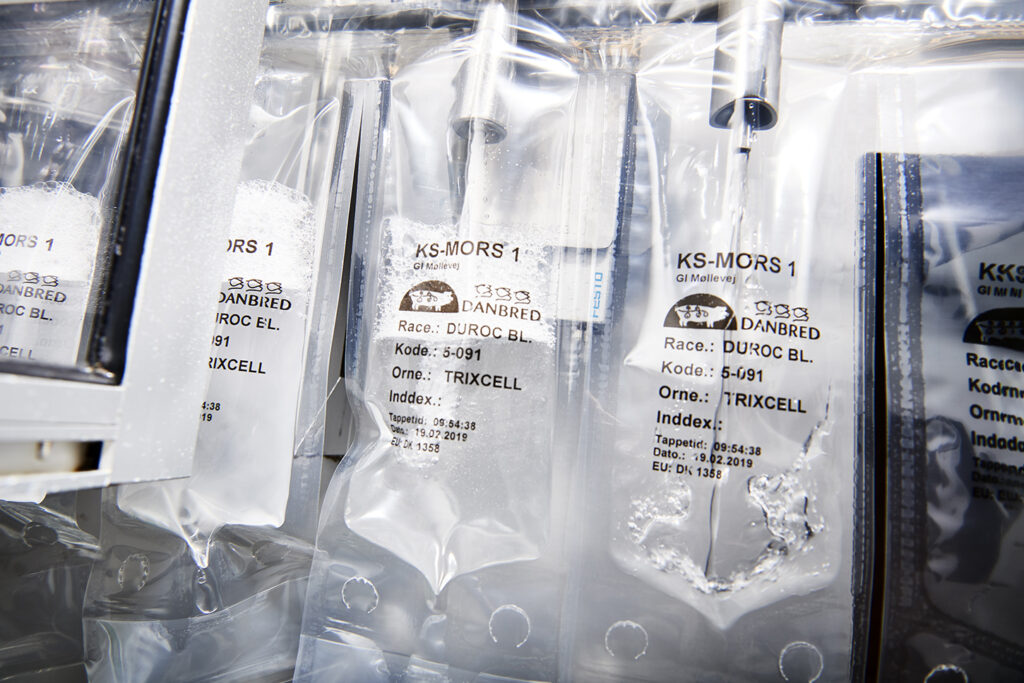 |
A normal AI dose contains 80-85 ml, including semen, extender and water.
|
|
|
Handling and storage of semen
Place the prepared semen doses in a correct storage facility directly after preparation, to preserve quality.
- Make sure you have a suitable cabinet for the storage of semen. The climate should be maintained at 16-18°C at all times.
|
|
|
Bear in mind the different temperature requirements at each step of processing prior to storage.
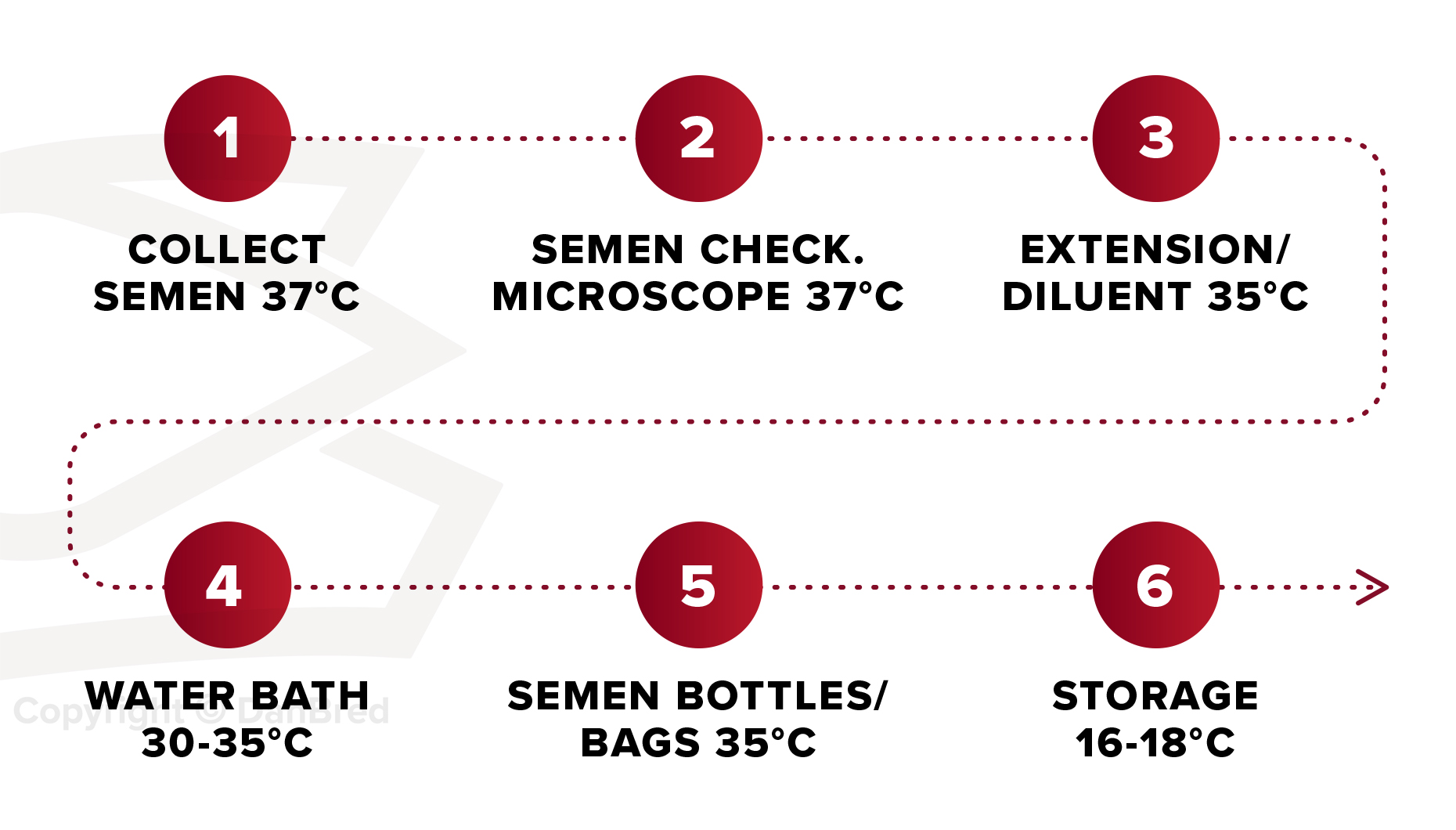
- Make sure that the semen is not heated or chilled before use.
- Keep semen doses out of direct sunlight.
- The semen doses should be turned over once or twice a day, as internally produced semen contains a higher concentration of sperm cells which can cause the sperm cells to suffocate.
 |
 |
- After storage and before use, make sure that all semen doses are controlled for viability so that the semen is alive and productive.
Checklist to assure the quality of semen in use
When working with high quality biological materials, it is essential to assure quality at every step of the way until farrowing to make sure that the semen delivers the expected results on farm:
1. Impaired production
- Swelling or atrophy of the testicles may occur, which suggests that semen production is impaired.
- Decreased feed intake will also decrease the production of semen, and the libido of the boar in general.
- Young boars will generally be less fertile. Boars younger than 220 days are still developing their sperm production.
2. Abnormal sperm cells
The boar produces semen, but a large amount of the sperm cells has deficits that causes returns, because they are unable to fertilise the oocytes.
- Deficits can be caused in periods with high ambient temperatures (more than 3 days).
- Deficits might be seen in boars older than 3 years which may have a growing number of abnormal sperm cells.
3. Acute disease and infections
Infertility may be caused by infections such as PRRS, Leptospira and Brucella Suis. Fever in particular can cause deficits in the sperm cells formed in the testicles. Deficits caused by fever can be permanent, but can also be temporary.
- If the temperature of a boar exceeds 40°C, it will have decreased sperm cell motility, which will result in decreased fertility rates. If a boar develops a fever, he should be quarantined for approximately two months before the next collection.
|
|
|
- Body temperature might increase immediately after vaccination, which should also be taken into consideration when planning the vaccination schedule.
- If the boar seems ill but no fever is registered, you should make a visual evaluation. Check the ejaculate for signs of abnormality- in particular if it contains blood, pus or excessive urine.
|
|
|
4. Excessive use of one boar
It is critical to follow the collection plan, as it ensures an even collection pattern amongst boars. Extensive use of one boar will cause a drop in sperm cell count, which will increase the amount of returns.
The number of returns among inseminated sows is the most important clinical sign of infertility in a boar. 21 days after insemination, rate the success. If returns are caused by infection, vulva discharge may occur in the sow.
 |
 |
Optimal environment to ensure quality of semen in use
Besides physical concerns with the boar itself or environmental matters, poor semen quality might occur due to environmental factors such as feed components, the quality of feed and water. Therefore there are many parameters which must be skilfully mastered when producing high-quality semen on farm.
1. Mycotoxins in the feed and/or straw can affect both the motility and morphology of the sperm cells.
- Analyse the feed and straw to rule out the feed components.
- A mycotoxin binder in the feed can be helpful, especially during high-risk periods such as immediately after harvest or if the feeding storage is not set up correctly.
- Remove old feed from the troughs prior to feeding to avoid feed residue containing mould and other potential contaminants.
2. Insufficient water flow will result in the boar drinking less, which affects the production and quality of semen. Water flow should be a minimum of 4 L/min for the boars to be able to drink enough.
3. An ambient temperature higher than 22°C will lead to an increased body temperature, resulting in reduced motility of the sperm cells.

















